Known CVD & T2D: See Access Info
Known CVD & T2D: See Access Info

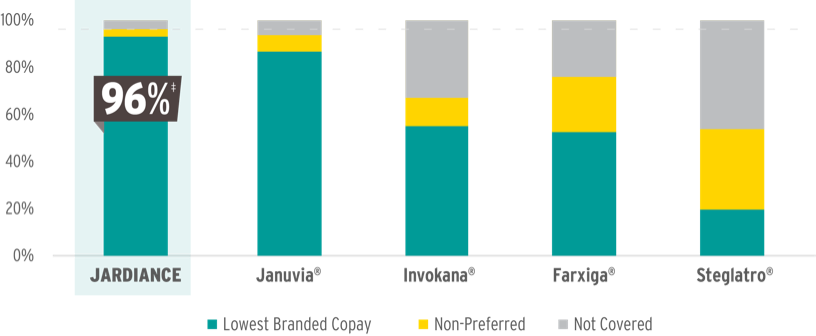
MORE PATIENTS HAVE ACCESS TO JARDIANCE
than all DPP-4is or SGLT2is across Commercial and Medicare Part D*
reduce the risk of CV death1
Jardiance is not for treatment of type 1 diabetes or diabetic ketoacidosis.
*Source: Fingertip Formulary, health plan or state listed above, and/or data on file, Boehringer Ingelheim Pharmaceuticals, Inc. as of 8/1/2019. Placement on formulary does not establish clinical comparability of products, including safety and efficacy, and is not a guarantee of full or partial coverage and/or payment. Contact health plan, state, or www.medicare.gov for most current information, as it may change without notice. This is not intended to be an exhaustive list of all plans in your area. Boehringer Ingelheim Pharmaceuticals, Inc. and Lilly USA, LLC do not sponsor or endorse any particular plan, and the company/plan names listed do not imply their endorsement of Boehringer Ingelheim Pharmaceuticals, Inc., Lilly USA, LLC, or the product(s) referenced.
Boehringer Ingelheim Pharmaceuticals, Inc. either owns or uses the trademark Jardiance® under license. Other referenced trademarks are owned by third parties.
†Percentage of covered lives represent a weighted average of both commercial and Part D availability.
‡Represents overall coverage.
ACC=American College of Cardiology; CV=cardiovascular; DPP-4i=Dipeptidyl Peptidase-4 Inhibitor; SGLT2i=Sodium-glucose Cotransporter 2 Inhibitors.
Reference: 1. Das SR, Everett BM, Birtcher KK, et al. 2018 ACC Expert Consensus Decision Pathway on Novel Therapies for Cardiovascular Risk Reduction in Patients With Type 2 Diabetes and Atherosclerotic Disease. J Am Coll Cardiol. Electronically published on November 26, 2018.
INDICATIONS AND LIMITATIONS OF USE
JARDIANCE is indicated to reduce the risk of cardiovascular (CV) death in adults with type 2 diabetes mellitus and established CV disease.
JARDIANCE is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
JARDIANCE is not recommended for patients with type 1 diabetes or for the treatment of diabetic ketoacidosis.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS: History of serious hypersensitivity to empagliflozin or any of the excipients in JARDIANCE; severe renal impairment, end-stage renal disease, or dialysis.
WARNINGS AND PRECAUTIONS
Hypotension: Empagliflozin causes intravascular volume contraction and symptomatic hypotension may occur. Before initiating JARDIANCE, assess and correct volume status in the elderly, and in patients with renal impairment, low systolic blood pressure, or on diuretics. Monitor for hypotension.
Ketoacidosis: Ketoacidosis, a serious life-threatening condition requiring urgent hospitalization, has been identified in patients with type 1 and type 2 diabetes mellitus receiving SGLT2 inhibitors, including empagliflozin. Fatal cases of ketoacidosis have been reported in patients taking empagliflozin. Patients who present with signs and symptoms of metabolic acidosis should be assessed for ketoacidosis, even if blood glucose levels are less than 250 mg/dL. If suspected, discontinue JARDIANCE, evaluate, and treat promptly. Before initiating JARDIANCE, consider risk factors for ketoacidosis. Patients may require monitoring and temporary discontinuation in situations known to predispose to ketoacidosis.
Acute Kidney Injury and Impairment in Renal Function: Empagliflozin causes intravascular volume contraction and can cause renal impairment. Acute kidney injury requiring hospitalization and dialysis have been identified in patients taking SGLT2 inhibitors, including empagliflozin; some reports involved patients younger than 65 years of age. Before initiating JARDIANCE, consider factors that may predispose patients to acute kidney injury. Consider temporary discontinuation in settings of reduced oral intake or fluid losses. Monitor patients for signs and symptoms of acute kidney injury. If it occurs, discontinue JARDIANCE and treat promptly.
Empagliflozin increases serum creatinine and decreases eGFR. Patients with hypovolemia may be more susceptible to these changes. Before initiating JARDIANCE, evaluate renal function and monitor thereafter. More frequent monitoring is recommended in patients with eGFR <60 mL/min/1.73 m2. Discontinue JARDIANCE in patients with a persistent eGFR <45 mL/min/1.73 m2.
Urosepsis and Pyelonephritis: Serious urinary tract infections including urosepsis and pyelonephritis requiring hospitalization have been identified in patients receiving SGLT2 inhibitors, including empagliflozin. Treatment with SGLT2 inhibitors increases the risk for urinary tract infections. Evaluate for signs and symptoms of urinary tract infections and treat promptly.
Hypoglycemia: The use of JARDIANCE in combination with insulin or insulin secretagogues can increase the risk of hypoglycemia. A lower dose of insulin or the insulin secretagogue may be required.
Necrotizing Fasciitis of the Perineum (Fournier's Gangrene): Serious, life-threatening cases have occurred in both females and males. Assess patients presenting with pain or tenderness, erythema, or swelling in the genital or perineal area, along with fever or malaise. If suspected, institute prompt treatment and discontinue JARDIANCE.
Genital Mycotic Infections: Empagliflozin increases the risk for genital mycotic infections, especially in patients with prior infections. Monitor and treat as appropriate.
Hypersensitivity Reactions: Discontinue JARDIANCE, treat promptly, and monitor until signs and symptoms resolve.
Increased Low-Density Lipoprotein Cholesterol (LDL-C): Monitor and treat as appropriate.
MOST COMMON ADVERSE REACTIONS (≥5%): Urinary tract infections and female genital mycotic infections.
DRUG INTERACTIONS: Coadministration with diuretics may enhance the potential for volume depletion.
USE IN SPECIAL POPULATIONS
Pregnancy: JARDIANCE is not recommended, especially during the second and third trimesters.
Lactation: JARDIANCE is not recommended while breastfeeding.
Geriatric Use: JARDIANCE is expected to have diminished efficacy in elderly patients with renal impairment. Renal function should be assessed more frequently in elderly patients. The incidence of volume depletion-related adverse reactions and urinary tract infections increased in patients ≥75 years treated with empagliflozin.
CL-JAR-100023 10.30.18
Please see JARDIANCE Prescribing Information and Medication Guide.


Copyright © 2019 Boehringer Ingelheim Pharmaceuticals, Inc. All rights reserved.
(08/19) PC-US-108372
